Permanganate Aerobic oxidation of Drimarene Blue-HFRL under diverse pH and impact on Chemical oxygen demand of the dye wastewater
| Received 04 Dec, 2023 |
Accepted 20 Feb, 2024 |
Published 28 Mar, 2024 |
This study aimed to explore the oxidation kinetics of reactive Drimaren Blue-HFRL (DB-HFRL), leading to its degradation by permanganate ions under diverse pH conditions at λmax610nm using a spectrophotometer. The oxidation reaction of DB-HFRL was observed on several parameters in acidic and alkaline solutions, such as dye and potassium permanganate (KMnO4) dosage, pH, reaction time, and temperature. The oxidative products were analyzed through IR spectroscopy, followed by the determination of chemical oxygen demand (COD) and biological oxygen demand(BOD) of reaction mixtures. The change in pH on the oxidation capability of KMnO4 was monitored under mono, di, and tri protic acids and an alkaline medium. Results showed that oxidation leading to degradation was higher at low pH compared to higher pH. The percent decolorization/ oxidation of dye waste effluent was rapid at higher concentrations of KMnO4 in the presence of different acids such as CH3COOH, H2SO4,and H3PO4 (76.19%) while minimum in alkaline medium (17.19%). It was observed that the dye decolorization rate was directly proportional to the hydrogen ion concentration in a given acidic medium (tri-protic acid, H3PO4). This indicates that H ions are significant in dye colour removal and renovation of wastewater. COD of the laboratory scale prepared wastewater (4500mg/L) showed the effectiveness of the developed process where a remarkable decline in chemical oxygen demand (COD) was observed in an acidic medium. This study establishes a cost-effective method to treat textile effluent to protect the ecosystem.
INTRODUCTION
Of the various industrial wastewaters, those discharged from the dyestuff and textile industries are the most difficult to treat. Wastewater from such industries is sometimes known as dye wastewater. Alkalinity, biological oxygen demand (BOD), chemical oxygen demand (COD), and dissolved solids with a concentration of dyes under 1.0 g.dm-3 are generally the characteristics of such wastewater[1,2]. The complex aromatic structure and the synthetic origin of dyes make them stable and resistant to biodegradation[3,4]. These dyes are divided into three distinct types: anionic dyes, which are direct and reactive; cationic dyes, which are basic; and non-ionic dyes, which are made of azo groups or anthraquinone types. Of these three, non-ionic or anthraquinone-based dyes are the most degradation-resistant. They have fused aromatic structures, which give these dyes the ability to resist degradation[4]. It is alarming that azo-based dyes are being used extensively and account for 60% to 70% of the whole dye structures known to be manufactured[5]. Versicolor has been used for dye decolorization, and it has been found that decolorization was dependent on the dye structures[6]. Textile industries use a lot of water[3]. Initially, they used the water as steam for heating purposes. Then, the water is used as a medium to transfer dyes to the fibers. The use of water is just not limited to these two processes. It is also required for the processing of the fibers. One of the widely used fibers is cotton, a substrate that requires a lot of water during its processing. 70 to 150 litres of water is consumed by dyeing 1 kilogram of cotton with reactive dyes.
Additionally, 0.6 to 0.8 kilograms of sodium chloride (NaCl) and 30 to 60 grams of dyestuff are used in this dyeing process. The high salinity of the effluents is the result of the electrolytes mentioned above. The pollution caused by using the dyes can be estimated from the fact that more than 80,000 tonnes of anionic (reactive dyes) are produced and consumed annually[7]. The wastewater generated using reactive dyes mainly contains hydrolyzed reactive dyes, not fixed on the substrate. It represents 20 to 30 percent of the reactive dyes applied (on average 2 g.L-1). This residual amount is non-recyclable and is responsible for the coloration of the effluents[8]. In addition, the wastewater also contains non-recyclable dyeing auxiliaries or organic materials (responsible for high BOD / COD of effluents), textile fibers, 60 g.L-1 to 100 g.L-1 of the electrolyte. This electrolyte is mainly NaCl and sodium carbonate (NaCO3)[9]. The dye wastewater creates water high in COD and BOD that must be treated before discharge to protect the aquatic life[10,12]. Manganese (VII) is an effective oxidizing and hydrolyzing agent[12]. It has been used widely in synthetic and analytical chemistry for oxidation[13]. One advantage of using Manganese (VII) is that it is a self-indicator[14]. The oxidizing ability of this oxidizing agent is dependent upon the pH of the medium[15]. The expected species in an acidic medium are mainly Mn(VII), Mn (IV) and Mn (III)[16]. Furthermore, in an acidic medium, Mn(VII) species are HMnO4, H2MnO4+, Mn2O7 and MnO3+[17].
The explanation for the H+ dependence on the rate of MnO4- oxidation has been explained in terms of protonation of MnO4- in a fast step to give HMnO4, which then reacts with the reductant in a slow step to provide the products[18]. The redox reaction of malachite green and peroxydisulphate ion occurred in one one ratio, with first-order dependence on the reductant (malachite green) and fractional order (one-half) dependence on the oxidant (peroxydisulphate) and rates of reaction were enhanced under both high or low pH relative to neutral conditions[19]. Moreover, the dyeing process contributes as the primary source of colour in textile waste (effluents). Discharging coloured compounds and effluents from various industrial activities is unhealthy for the environment. In addition to that, the wastewater has an intense colour and high BOD / COD and TOC (total organic carbon) values. The worldwide annual production of dyestuff amounts to more than 7 x 105 tonnes[20]. Their breakdown products may be toxic, and their removal from the effluent is a major environmental problem and a need of today[21-23]. Due to the complex aromatic structures, these dyes are unaffected by light, temperature and microbial attack. Because of this, they are known as recalcitrant compounds[24,25].
The dye sewages are high in colour, pH, chemical oxygen demand (COD), and biological oxygen demand (BOD)[26-34]. Therefore, the aim and objective of the current investigation is to develop a treatment process which should monitor and compare these parameters with the standard concentrations before discharging. In this study, these critical parameters, pH, COD, and BOD, are taken into consideration to develop cost-effective and eco-friendly processes for successful control of wastewater to safe running streams.
MATERIAL AND METHODS
All reagents, potassium permanganate, acetic acid, sulphuric acid, phosphoric acid, and sodium hydroxide obtained from E- Merck, were used as received. A reactive dye, Drimaren Blue HFRL, was taken from the local textile industry. The experiment was divided into different sessions. Solutions were prepared according to experimental needs, and the reaction was monitored through spectral changes; absorbance was noted at wavelength 610nm, and kinetic measurements were done.
Preparation of Sample Solutions
The decoloration of Drim Blue HFRL was carried out by preparing it in de-ionized water, which was further diluted with de-ionized water to obtain a series of dye solutions with varying concentrations. The dye solution was scanned from 200 to 800 nm, and its maximum absorbance (λmax 610 nm) was determined. The concentration of a stock solution of KMnO4 was 0.002 M; reaction mixtures were obtained by taking an appropriate amount of stock dye solution, adding KMnO4 with adjusting pH, and transferring to cuvet. The 0.1 ml. of 1M HONH3Cl (Hydroxylammonium chloride) was added to eliminate the colour disturbance caused by residual KMnO4 to the absorbance measurement. HONH3Cl reacts with residual KMnO4 to produce a colourless compound. The absorbance value obtained in each case was plotted against time to get a rate constant.
pH and Kinetics Measurements
pH of the reaction mixture in diverse acids and alkaline conditions is measured through pH meters. Kinetics measurements were made by preparing three sets of reaction mixtures in which one species was varied while the others were kept constant at a given concentration. All contents were mixed, and the progress of the (inlets) reaction was monitored by recording the change in optical density during the reaction on a UV/Visible spectrophotometer (Shimadzu 160-A).The reaction order was evaluated by measuring the specific reaction rate at various pH[19-27]. The percent decrease in absorbance was calculated by using the formula:
| (1) |
where
| Af | = | absorbance after 15 min or final absorbance | |
| Ai | = | Absorbance at 0 min or initial absorbance |
Analysis of COD and BOD
The analysis of COD was conducted by COD analyzer APHA 5220-C. The amount of organic compounds present in the wastewater is determined by chemical oxygen demand, usually referred to as COD of the running stream. In acidic conditions with strong oxidizing agents, these compounds oxidize into CO2. In this method, different samples of reaction mixtures were refluxed for 2-3 hr in a strongly acid solution with a known additional amount of potassium dichromate (K2Cr2O7)[26]. The remaining unreduced K2Cr2O7 was determined by titration with ferrous ammonium sulphate, and the oxidizable matter was calculated in terms of oxygen equivalent. COD is expressed in mg/L, or ppm, and reflects the mass of oxygen consumed per litre of solution. This test is completed almost within 2-3 hr, while BOD requires five days[28]. Therefore, the following equation was used to estimate the BOD of the dye wastewater before and after the reaction as described by[28].
RESULTS AND DISCUSSION
Dye coloration is an essential step in the environmental monitoring of textile wastewater because of the detrimental effects associated with the colour water per several additives used in the textile industry to fix the colour on fabrics. Massive quantities of dye wastewater discharged after its primary use also impact running streams; consequently, COD and BOD of water reserves are affected. The oxidation leading to degradation of Reactive Drim Blue HFRL (DB-HFRL) was investigated in an aqueous medium using a powerful oxidizing agent, KMn4O, at several pH using diverse acids and alkaline medium followed by estimation of COD and BOD. Results showed that KMnO4 works most efficiently as a potent oxidizing agent in different acids related to H+ and alkaline medium, especially at low pH, due to which reduction of Mn (VII) (gain of e-) to Mn (II) on KMnO4 may take place quickly and thus become an excellent oxidizing agent (Fig.1). The generation of nascent oxygen; in the acidic medium also responsible for oxidizing the dye molecule leading to degradation.
At high pH, the reaction was prolonged because fewer electrons were transferred per mole permanganate, whereas Mn(VII) was reduced to manganite (VI). It was recognized through spectral studies that dye degradation occurs through complex formation, which dissociates into smaller products. The spectral analysis of the dye wastewater after reaction showed that the dye was oxidized entirely, and no peak was recorded at lmax 610nm.

|
Reaction Pathway of Decolouration DB-HFRL Under Various pH
KMnO4is applied as an oxidant to the dye wastewater as it is suitable to oxidize weak bonds such as π bond, aromatic ring, and C-H. The aerobic oxidation of DB-HFRL was monitored in an acidic (mono, di and triprotic acid) and alkaline aqueous medium using KMnO4, having high redox potential and act as a potent oxidizing agent at low pH, since it reduced to the highest degree in an acidic medium, from oxidation state (+7 ) in MnO−4 to (+2) in Mn2+ according to the following Equations:
| (2) |
| (3) |
| (4) |
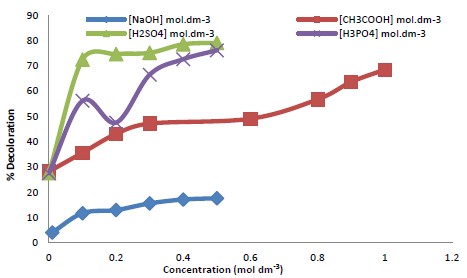
Equations (2-4) showed the oxidation potential of KMnO4 in different mediums with the diverse release of electrons. Equation (1) shows that one molecule of KMnO4 releases 5 electrons per species, which is high and reflects that a trivial amount of KMnO4 is enough for the oxidation reaction. Due to this, KMnO4 was selected as the best oxidizing agent for controlling hazardous dye wastewater for economic retorts. Therefore, oxidation reaction was studied using three different types of acids (Fig. 2) related to the proton number, and it was found that oxidation was proton-dependent (Table 1).
|
| Table 1: | Effect of Change in pH in relation to the concentration of NaOH, CH3COOH, H2SO4 and H3PO4 in the presence of KMnO4 (pH=2-14) | |||
[DB-HFRL] = 100 ppm;[KMnO4] = 2 x 10-5 mol.dm-3 |
||||
| S.No. | [NaOH] (mol.dm-3) |
Rate of Reaction (dx/dt) |
Rate constant “k” |
% Decoloration |
| 1 | 0.01 |
06.0×10-4 |
0.4699 |
4.16 |
| 2 | 0.1 |
2.0×10-3 |
0.4515 |
11.78 |
| 3 | 0.2 |
2.1×10-3 |
0.4484 |
13.02 |
| 4 | 0.3 |
1.9×10-3 |
0.4514 |
15.66 |
| 5 | 0.4 |
2.4×10-3 |
0.4447 |
17.19 |
| 6 | 0.5 |
2.5×10-3 |
0.4433 |
17.69 |
| S.No. | [CH3COOH] (mol.dm-3) |
Rate of Reaction (dx/dt) |
Rate constant “k” |
% Decoloration |
| 1 | 0 |
3.8×10-3 |
0.4169 |
28.24 |
| 2 | 0.1 |
5.0×10-3 |
0.3823 |
35.68 |
| 3 | 0.2 |
5.5×10-3 |
0.3613 |
43.12 |
| 4 | 0.3 |
6.0×10-3 |
0.34 |
47.22 |
| 5 | 0.6 |
7.0×10-3 |
0.32 |
49.12 |
| 6 | 0.8 |
8.2×10-3 |
0.3 |
56.78 |
| S.No. | [H2SO4] (mol.dm-3) |
Rate of Reaction (dx/dt) |
Rate constant “k” |
% Decoloration |
| 1 | 0 |
3.7×10-3 |
0.4096 |
27.61 |
| 2 | 0.1 |
9.7×10-3 |
0.2537 |
72.51 |
| 3 | 0.2 |
1.4×10-2 |
0.216 |
74.68 |
| 4 | 0.3 |
1.8×10-2 |
0.1782 |
75.18 |
| 5 | 0.4 |
2.1×10-2 |
0.1477 |
78.56 |
| 6 | 0.5 |
2.3×10-2 |
0.1235 |
80.23 |
| S.No. | [H3PO4] (mol.dm-3) |
Rate of Reaction (dx/dt) |
Rate constant “k” |
% Decoloration |
| 1 | 0 |
3.7×10-3 |
0.4096 |
27.61 |
| 2 | 0.1 |
7.4×10-3 |
0.3456 |
56.23 |
| 3 | 0.2 |
6.3×10-3 |
0.3636 |
47.59 |
| 4 | 0.3 |
1.24×10-2 |
0.2454 |
66.54 |
| 5 | 0.4 |
1.53×10-2 |
0.1912 |
72.66 |
| 6 | 0.5 |
1.88×10-2 |
0.1635 |
76.19 |
The result showed that the colour removal of dye increased in the acidic medium in the presence of KMnO4 than in alkaline. More than 90% decoloration was achieved with a maximum applied concentration of KMnO4 (i.e.: 0.06 - 0.1mM) following pseudo-first-order kinetics (Table 2).
The pH influences the processes used for dye wastewater treatment through biological or chemical methods. A literature search showed that a pH value less than (1.5) showed the decolorization efficacy was very high. In contrast, when pH was higher than 4.0, the dye decoloration was not completely decolorized in the presence of KMnO4[27]. It was observed that pH had a remarkable effect on the decolorization efficiency of the dye. The investigation established that the DB-HFRL was more persistent and resistant to degradation at high (alkaline) pH compared to low pH (acidic), i.e., the decolorization efficiency was very high(80.23%). In comparison, it was low in an alkaline medium (17.69%) (Fig. 3).

|
Moreover, it revealed that an acidic medium favours decolorization rather than an alkaline medium. It has already been reported that the dyes display diverse firmness at different pH levels, similar to the current observation. The less decoloration at high pH in the current investigation may related to the chemical structure of dyes, which can impact their vulnerability to decoloration at different pH levels. It may involve specific functional groups of the organic dye molecules that may be extra reactive or susceptible to degradation under particular pH conditions[25-32]. The pH also determines whether an oxidation reaction occurs via one, three, or five electron exchanges[30]. The current results are according to the previously published reports of Siddique et al.[26], who observed that the extent of decoloration was increased by decreasing pH followed by first-order kinetics (Table 2). Racyte et al.[27] investigated the effect of pH on real dye wastewater at a lab-scale reactor with the original high pH of the wastewater decolorized in 5 hours while at pH 3 (acidification), a speedy decolorization of the dye was observed.The concentration of KMnO4 had a significant effect on decolorization efficiency (Table 2) related to pH, where an increase in the concentration of the reducing agent showed an increase in % decoloration(99.89%) when triprotic acid was used.
| Table 2: | Effect of Change in Conc. of KMnO4 in the presence of NaOH, CH3COOH, H2SO4 and H3PO4 | |||
[NaOH] =[CH3COOH] =[H2SO4] =[H3PO4] 0.1mol.dm-3,[DBHFRL] = 100 ppm |
[NaOH] = 0.1mol.dm-3 |
|||
| S.No. | [KMnO4] x 105 (mol.dm-3) |
Rate of Reaction (dx/dt ×10-3) |
Rate constant “k” |
% Decoloration |
| 1 | 0 |
1.4 |
0.52 |
11.21 |
| 2 | 2 |
2 |
0.4515 |
11.78 |
| 3 | 4 |
3 |
0.3827 |
13.12 |
| 4 | 6 |
3.8 |
0.3412 |
15.16 |
| 5 | 8 |
4.4 |
0.3126 |
17.71 |
| 6 | 10 |
4.9 |
0.2876 |
19.23 |
[CH3COOH] = 0.1mol.dm-3 |
||||
| S.No. | [KMnO4] x 105 (mol.dm-3) |
Rate of Reaction (dx/dt) |
Rate constant “k” |
% Decoloration |
| 1 | 0 |
0.8×10-5 |
0.476 |
1.613 |
| 2 | 2 |
9.2×10-3 |
0.232 |
56.61 |
| 3 | 4 |
1.06×10-2 |
0.187 |
71.45 |
| 4 | 6 |
1.23 ×10-2 |
0.008 |
87.34 |
| 5 | 8 |
1.37×10-2 |
0.006 |
91.31 |
| 6 | 10 |
1.49×10-2 |
0.005 |
93.65 |
[H2SO4] = 0.1mol.dm-3 |
||||
| S.No. | [KMnO4] x 105 (mol.dm-3) |
Rate of Reaction (dx/dt) |
Rate constant “k” |
% Decoloration |
| 1 | 7.0×10-3 |
0.339 |
53.66 |
|
| 2 | 2 |
9.0×10-3 |
0.253 |
72.32 |
| 3 | 4 |
1.2×10-2 |
0.052 |
94.36 |
| 4 | 6 |
1.3×10-2 |
0.141 |
98.31 |
| 5 | 8 |
1.5×10-2 |
0.122 |
98.56 |
| 6 | 10 |
1.7×10-2 |
0.101 |
99.12 |
[H3PO4] = 0.1mol.dm-3 |
||||
| S.No. | [KMnO4] x 105 (mol.dm-3) |
Rate of Reaction (dx/dt) |
Rate constant “k” |
% Decoloration |
| 1 | 0 |
3.7×10-3 |
0.414 |
28.12 |
| 2 | 2 |
7.4×10-2 |
0.345 |
56.23 |
| 3 | 4 |
1.12×10-2 |
0.139 |
85.1 |
| 4 | 6 |
1.32×10-2 |
0.332 |
99.31 |
| 5 | 8 |
1.39×10-2 |
0.126 |
99.56 |
| 6 | 10 |
1.57×10-2 |
0.112 |
99.89 |
The low oxidation at higher pH may be explained as their low oxidation potential related to the oxidation state of Mn according to the equation (1-3). It was observed that the oxidation potential (Eo) increases with decreasing pH. Equations 2 & 3 showed that Eo in an acidic solution is much higher than in an alkaline (Equation 3) solution.
Mechanism of oxidation
Monoprotic acid
| (5) |
Diprotic acid
| (6) |
Triprotic acid
| (7) |
| (8) |
| (9) |
Oxidation reaction(eq. 5-9) showed a reduction of MnO-4 via 3 to 6 e- and validated that in triprotic acid, KMnO4 acts as a strong oxidizing agent for dye removal. The highest oxidation in minimum time may be explained in relation to the structure of the reactive dyes in regard to the presence of the aromatic ring having a conjugated double bond, which can easily broken in different acidic mediums by KMnO4 according to the above equations (1-8). The results showed that the oxidation mechanism of DB-HFRL by permanganate ions in different mediums involved the attack of permanganate oxidants on the center of the substrate DB-HFRL, giving an intermediate complex (C1) which later on degraded into colourless smaller components according to the following equation.
| (10) |
Chemical Oxygen Demand and Biological Oxygen Demand
COD and BOD were measured to determine sludge quality and detect the industrial discharge by applying safe, low-cost technology to prevent environmental pollution for the safety of the running streams. Chemical oxygen demand is a vital water quality parameter, measuring the quality of sustainable marine life. It is frequently used for the biological oxygen demand (BOD) of waterbodies for life in water streams. BOD provides an index to evaluate the effect of discharged wastewater which the running streams receive when dye waste is discharged. The measurement of COD of dye wastewater reflects the organic/inorganic compounds used in dye fabrication and discharged in water bodies, which contributes to the contaminates. COD is the quantity of oxygen expended to chemically oxidize organic water pollutants to inorganic end products, i.e., water and CO2. Therefore, the COD test was applied to measure the strength of organic wastes. It is the application that is used to determine organic compounds in water. Higher COD levels (Table 3) indicate a quantity of oxidizable organic substances in the water sample, responsible for reducing dissolved oxygen (DO) levels; consequently, a reduction in dissolved oxygen (DO) appeared as an anaerobic condition, which is toxic to higher aquatic life forms.
| Table 3: | COD and BOD of different reaction mixtures studied | |||
| Several studied Reactions | COD (ppm) |
Permissible limit (ppm) |
BOD(ppm) |
Permissible limit (ppm) |
| Dye wastewater | 4500 |
17,620.02 |
||
| KMnO4 neutral | 900 |
936.9042 |
||
| KMnO4/CHCOOH | 150 |
250 to 500[32] |
71.2542 |
30[32] |
| KMnO4 /H2SO4 | 80 |
300–600 ppm[33] |
36.3802 |
150–300 ppm[33] |
| KMnO4 /H3PO4 | 65 |
29.9272 |
||
| KMnO4 /NaOH | 200 |
100.9642 |
In this test, a potent oxidizing agent like K2Cr2O7 under acidic conditions was applied to the reaction mixture of dye and oxidant in different acidic conditions (H+ ions) and an alkaline medium. It was assessed based on the fact that a strong oxidizing agent, under acidic conditions or alkaline medium, can practically oxidize entire organic compounds to carbon dioxide and water. Results showed that dye wastewater has higher COD and unbalanced BOD without treatment while having less (Table 3) after applying the trial compared to the permissible limit and domestic, respectively (Table 3). The current results are in accordance with the reports where BOD is less than COD[28]. The low COD after the trial validated that the applied method is innovative in controlling the dye wastewater compared to the earlier work[28], where KMnO4 as an oxidizing agent is suitable in acidic conditions to control hazardous colouring water. Results are according to the previously published report of Inam et al.[34], who observed excellent decolorization followed by an extreme decline in COD at a high temperature of 60oC. At the same time, the current investigation proves to be innovative as COD is observed at normal temperatures.
CONCLUSION
It was concluded that the decolorization rate of DB-HFRL dye solutions in several acidic mediums was rapid as compared to the alkaline medium, which was linked with the oxidation potential of the oxidant. It was established that pH plays a vital role in dye wastewater management, followed by reducing COD of the dye wastewater. Results showed that the maximum decoloration was obtained at pH 1.5; however, more research is recommended to check the final pH of the recovered water before discharging it into the running stream as an essential step.
ACKNOWLEDGEMENT
We are thankful to PCSIR laboratories and the Department of Chemistry for providing research facilities to compile this Ph.D research project
REFERENCES
- A. W. Waithaka. Wastewater Management in Cotton Wet Processing in Thika Cloth Mills in Kiambu County, Kenya (Doctoral dissertation, Kenyatta University) (2017).
- C. A. GalarzaVillalba. (Bachelor'sthesis, UniversidadTécnica de Ambato. Facultad de Ingeniería Civil y Mecánica. Carrera de Ingeniería Civil) (2017).
- H., Emgili, E., Yabalak, Ö., Görmez, & A. M. Gizir. Gazi University Journal of Science, 30(4), 140-150 (2017).
- C., Nwodika, & D. O. ONUKWULİ. Gazi University Journal of Science, 30(4), 86-102. (2017).
- L., Song, Y., Shao, S., Ning, & L. Tan. Bioresour. Technol., 233, 21-29. (2017).
- A. C., amillo, M., Cobas, A., Hormaza, & M. Á. Sanromán. Water, Air, & Soil Pollution, 228(6), 205. (2017).
- A., Rai, P. S., Chauhan, & S. Bhattacharya. Water Remediation, 171-187. (2018).
- X., Liang, Y., Lu, Z., Li, C., Yang, C., Niu, & X. Su, Microporous Mesoporous Mater., 241, 107-114. (2017).
- I. M. S., Pillai, & A. K. Gupta. J. Environ. Manage., 193, 524-531. (2017).
- S. P., Kodal, & Z. Aksu, Environ. Technol., 1-11. (2017).
- N. A., Oladoja, E. I., Unuabonah, O. S., Amuda, & O. M. Kolawole. Polysaccharides as a Green and Sustainable Resources for Water and Wastewater Treatment, 1-11. (2017).
- K., Yin, F., Li, Y., Wang, Q., He, Y., Deng, S., Chen, & C. Liu. J. Hazard. Mater., 330,52-60. (2017).
- M., Risch, K. A., Stoerzinger, B., Han, T. Z., Regier, D., Peak, S. Y., Sayed, & Y. Shao-Horn. The Journal of Physical Chemistry C, 121(33), 17682-17692. (2017).
- F., Gashi, S., Frančišković-Bilinski, H., Bilinski, A., Rexhepi, & A. Sustainable Water Resources Management, 3(1), 1-12. (2017).
- C. N., Butterfield, & B. M. Tebo. Metallomics, 9(2),183-191. (2017).
- H. Boumaiza, R. Coustel, G. Medjahdi, C. Ruby, L. & L. Bergaoui. J. Solid State Chem., 248, 18-25. (2017).
- S. Ruggeri, F. Terzi, B. Zanfrognini, E. Corsi, N. Dossi, C. Zanardi, & R. Seeber. Electrochim. Acta., 240, 108-113. (2017).
- M. Zhang, Q. Gao, C. Yang, L. Pang, H. Wang, R. Li, H. Yang, R. Li, Z. Xing, J. Hu, & G. Wu. Appl. Surf. Sci., 422, 1067-1074. (2017).
- H. Hosseinzadeh, & S. Ramin. Int. J. Biol. Macromol., 106, 101-115. (2018).
- A. A. Prasada, G. Kumara, & D. M. Thomasb. Bull. Chem. Pharma Res, 1(1), 30-39. (2017).
- M. S. Arif, & S. R. Malik. NFC IEFR J. Eng. and Sci. Research, 5. (2017).
- F. D. Castro, j. P. Bassin, & M. Dezotti. Environ. Sci. Pollut. Res., 24(7), 6307-6316. (2017).
- K. Meerbergen. Decoloration of Textile Wastewater, with an Emphasis on Microbial Treatment Processes Thesis (2018).
- M. A. Kumar, P. Baskaralingam, A. R. S. Aathika, S. & S. Sivanesan. Waste Bioremediation, 165-183. (2018).
- S. M. Burkinshaw & G. Salihu. Dyes Pigm. (2019).
- M. Siddique, R. Khan, A. K. Khan, & R. Farooq. J. Chem. Soc. Pak., 36(1). (2014).
- X. R. Xu, H. B. Li, W. H. Wang, & J. D. Gu, Chemosphere,59(6), 893-898. (2005).
- J Abdulla, H., K Bashar Al-Quraeshi, N., Nabeeh, F., & J.-Awadi, J. Kerbala Uni.8(1), 40-43. (2012).
- J. Racyte, M. Rimeika, & H. Bruning Environ. Prot. Eng,35(3), 167-178. (2009).
- A. Yadav, S. Mukherji, & A. Garg.Ind. Eng. Chem. Res., 52(30), 10063-10071. (2013).
- Y. Gao, J. Jiang, Y. Zhou, S. Y. Pang, J. Ma, C. Jiang, & J. Li. Chem. Eng. J., 327, 418-422. (2017).
- https://www.envirozyme.com/resources/case-studies/envirozyme-eliminates-foam-in-municipal-wastewater-trial.
- L. B. Trevizani, A. Nagalli, F. H. Passig, k. Q. de Carvalho, G. J. Schiavon, & A. N. de Lima.Acta Scientiarum. Technology,40. (2018).
- I. Ullah, S. Ali, & M. Akram.Int. J. Chem. Biochem. Sci,4, 96-100. (2013).
How to Cite this paper?
APA-7 Style
Farhan,
M., Ahmed,
T., Manzoor,
S., Ejaz,
M. (2024). Permanganate Aerobic oxidation of Drimarene Blue-HFRL under diverse pH and impact on Chemical oxygen demand of the dye wastewater. Pakistan Journal of Chemistry, 14(1-2), 1-7. https://doi.org/10.15228/2024.v14.i1-2.p01
ACS Style
Farhan,
M.; Ahmed,
T.; Manzoor,
S.; Ejaz,
M. Permanganate Aerobic oxidation of Drimarene Blue-HFRL under diverse pH and impact on Chemical oxygen demand of the dye wastewater. Pak. J. Chem. 2024, 14, 1-7. https://doi.org/10.15228/2024.v14.i1-2.p01
AMA Style
Farhan
M, Ahmed
T, Manzoor
S, Ejaz
M. Permanganate Aerobic oxidation of Drimarene Blue-HFRL under diverse pH and impact on Chemical oxygen demand of the dye wastewater. Pakistan Journal of Chemistry. 2024; 14(1-2): 1-7. https://doi.org/10.15228/2024.v14.i1-2.p01
Chicago/Turabian Style
Farhan, M., T. Ahmed, S. Manzoor, and M. Ejaz.
2024. "Permanganate Aerobic oxidation of Drimarene Blue-HFRL under diverse pH and impact on Chemical oxygen demand of the dye wastewater" Pakistan Journal of Chemistry 14, no. 1-2: 1-7. https://doi.org/10.15228/2024.v14.i1-2.p01

This work is licensed under a Creative Commons Attribution 4.0 International License.


