A Review of the Significant Role of Heavy Metals in the Advancement of the World and its Contribution to Pollution
| Received 21 Jun, 2024 |
Accepted 18 Sep, 2024 |
Published 24 Sep, 2024 |
Heavy metals are transition elements belonging to the d-block of the periodic Table found in nature as a part of the earth's crust. They are recognized as having high atomic weight and density and having a substantial role in most fields of advancement, including industry, agricultural business, pharmaceuticals, and the environment. The current advancement of the world is thankful for these elements called heavy metals as they are a significant part of the scientific revolution. However, their diverse roles in the technological field raised concerns about plants, humans and environmental issues when placed in an open environment after their prime use without prior treatment or as leftovers. The heavy metals reactivity is due to the multiple oxidation states where electrons in a partially filled d orbital are available for combination with the nearest available matter, which may be heavy metal, inorganic or organic compounds. They lead to variations in environmental biota and appear as toxic materials, promoting pathogenic bacteria or polluting water and soil resources. This review highlights heavy metals' significant role in advancement, the necessity for progress, and their environmental toxicity.
INTRODUCTION
Heavy metals, referred to as d-block transition elements with diverse oxidation states due to partially filled "d" orbit, showed unique properties. Oxidation is a chemical reaction involving an electron transfer from an electron-rich to an electron-deficient unit. The electron-deficient element or molecule is termed an oxidizer or oxidizing agent. Due to vacant d-orbital, heavy metals are potent oxidizing agents[1-10]. Metals and metal compounds are natural environmental components affecting biological and nonbiological systems. Several studies have reported toxic and carcinogenic effects induced when plants, humans and animals are exposed to certain metals, especially arsenic (As), lead (Pb), cadmium(Cd), chromium(IV) and mercury(Hg). Many results prove that toxic and carcinogenic metals can interact with nuclear proteins and deoxyribonucleic acid (DNA), triggering the oxidative decline of biochemical macromolecules[3-11]. Detailed studies in the past two decades proved that metals like iron (Fe), Cd, Cr, Hg, As, and Pb possess the ability to produce reactive free radical species, which start a chain reaction resulting in the oxidation of lipids termed lipid peroxidation, protein oxidation and oxidation of active biomolecules like nucleic acids, cellulose, DNA and ribonucleic acid (RNA). Toxic free radical-mediated oxidations occur in aerobic organisms due to normal oxygen metabolism. Heavy metals such as Cd, Pb, As, and Hg play significant roles in daily life but have no biological roles in living organisms. Their impact is evident in numerous scientific fields, including industry, health, environmental science, and technology. However, releasing these metals into the open atmosphere makes them toxic to living organisms, including plants and animals[1,6-10].
The Role of Heavy Metals in the Industrial Revolution
Heavy metals are a revolutionary part of the advancement of the world. In almost every field of science and technology, their indispensable presence is highly appreciated and can't be ignored. For example, Pb is used to manufacture several types of batteries, radiation shielding, and ammunition[6]. Hg is a part of the pharmacological industry used in thermometers and dental amalgams[7].
Electronics and Batteries
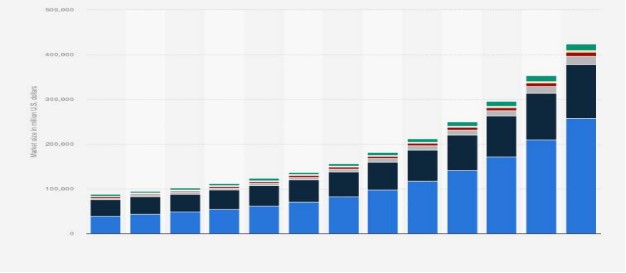
We have all been familiar with battery technology for a long time; e.g. home speakers, smoke detectors, clocks, cameras, tools and household appliances are in continued use. Inside healthcare, a great deal of vital equipment is powered by batteries, like hearing aids, insulin pumps, pacemakers and defibrillators[9]. The daily use of electronics and batteries mainly depends upon three metals: Cd, Pb, and Ni. Global battery market size by technology is shown in Fig.(1). These metals are also used in manufacturing industrial items such as car, truck, and bus batteries[10]. For example, nickel-cadmium and lead-acid batteries are widely used in electronic devices, automobiles, and renewable energy storage systems[11-14]. Due to their excellent conductive properties, heavy metals like Ni, Fe, and Cu are essential in electronics for wiring, connectors, and printed circuit boards. Metals, therefore, have a vital role to play in both energy efficiency and energy storage[12]. Copper is required to achieve the E.U.'s goal of 30 percent increased energy efficiency by 2030, and various metals are needed to enable energy storage to a much greater extent than is currently the case.[13,14].
|
The role of heavy metals in Construction and Manufacturing:
Lead (Pb) is used in the construction industry due to its elasticity to form roofs and other covering plates, windows, linings for valances, tanks, headers, gutters and drainpipes, alternating, and a component of soft solder. It is also used in building materials, like paints, although its use has declined due to health concerns[15]. Chromium, often found in stainless steel, is critical for manufacturing durable and corrosion-resistant materials used in various applications, including kitchen utensils, medical instruments, and building structures[16]. Cuis malleable, non-magnetic stable, light, soft, with little maintenance metal, high thermal and electrical conductivity, and corrosion-resistant.Cu is influential in the construction industry, as it is applied in pipe forming and tubing forwaterdistribution.Cu is also used to make electrical and communication cables, which are generally recyclable, and this, combined withitslonglife,has relatively lowlife cycleimpacts[17]. Iron (Fe) is a widespread element commonly used in variousarchitecturalapplications because ofitsrelativeaffordability[18]. Today,aluminum(Al) is the second most usedmetalinbuildingsforroofing,flashing, wallpanels,and spandrels aftersteel[19] (Fig.2).

|
The role of heavy metals in the synthesis of Pigments and Dyes
Metal-complex dyes and pigments are formed by coordinating bi- or polyvalent transition metal ions with diverse dyes. They have an extensive range of applications in various fields, including the dyeing of nylon and protein fibers, paint, toners for photocopiers, laser and ink-jet printers, photoconductors for laser printers, nonlinear optics, singlet oxygen generators, dark oxidation catalysts, and high-density memory storage devices[21].
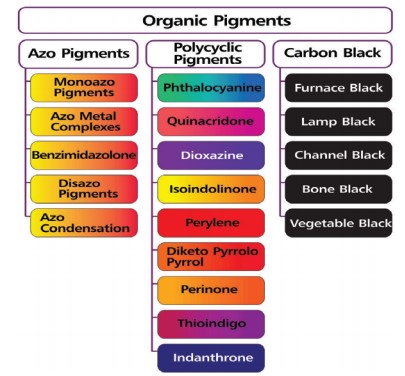
Pigments are organic or inorganic compounds insoluble in oil, acrylics, or tempera and dispersed within the paint medium (Fig.3). The lack of solubility aids in keeping the pigment fixed in its place once the paint is applied. Heavy metals like Cr and Cd produce vibrant pigments for paints, plastics, ceramics, and glass. Many inorganic pigments that contain heavy metals (Pb, Cd, Hg) are toxic. While they pose little threat once fixed in a dried medium on a painted object, preparing such pigments as fine powders exposes the painter or the manufacturer to toxic dust, and modern, commercially prepared paints generally avoid highly poisonous compounds. Lead paints used in houses are tarnished for contaminating soils when the paints are scraped off during re-painting[21,23]
|
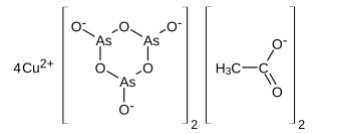
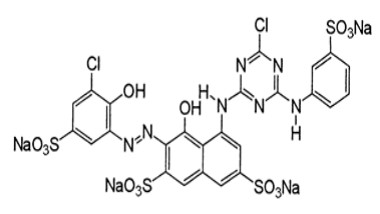
Dye molecules are soluble in a suitable solvent, most often water, to facilitate the dyeing process to which the textile is added. Most dyes are organic molecules, but some do contain inorganic materials. An infamous example is Paris green (Fig.4), an arsenic-containing compound used for dyeing fabrics for clothing in the 1800s. An important consideration, especially with organic dyes, is the molecules' response to pH. Many change colour depending on the solution's acidity and find application as acid-base indicators in the laboratory. Indigo is a substantive dye, while cochineal is an adjective dye. The role of the mordant is elementary. It is usually a metal ion capable of binding to functional groups in the organic molecules of the textile and functional groups in the dye molecule. Common mordants included alum (KAl[SO4]2·12H2O) and other Al, Cr, Cu, Fe, K, Na and Sn salts[24-30]. The binding mechanism to the metal occurs through lone pairs of electrons on functional groups in the organic molecules. A cellulose fragment, an Al cation and a cochineal dye illustrate this. The transition metal ions such as Cr, Cu and Fe would possess their colour from the electronic transitions in the d-orbitals (Fig.5 & 6). They would impact the dyed material's end colour. Metal-complex dyes are usually used on wool, silk, and nylon to achieve superior wash fastness in the dyed fabrics compared to those treated with the original acid dye[31-33].
|
|
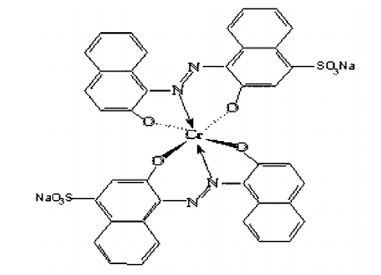
|
Metal as Catalysts in Chemical Reactions
Transition metals act as appropriate catalysts; they can exist in two or different oxidation states accomplished exhibiting several oxidation states and acting as oxidizing and reducing agents, e.g. FeO (II) oxide and Fe2O3 (III) oxide. Metal catalysts are widely used in numerous reactions as these can adsorb dissociative molecules and provide a surface to allow the fragments to react together and desorbed. Metals like Fe, Ni, Mo, and Co regularly assemble nanomaterials[30]. Platinum (Pt) and palladium(Pd) metals are crucial in catalytic converter reactions called 'oxidation catalysts' that convert hydrocarbons (harmful vehicle emissions) into water and carbon monoxide into carbon dioxide[30-34]. Furthermore, Palladium, used with rhodium, is also known as a 'reduction catalyst,' which converts NOx into less toxic nitrogen compounds, elemental nitrogen, and oxygen[30-36] (Fig.7 & 8). Metal catalystsare recognized as the most critical factors in accelerating lipid oxidation in dairy products[32].
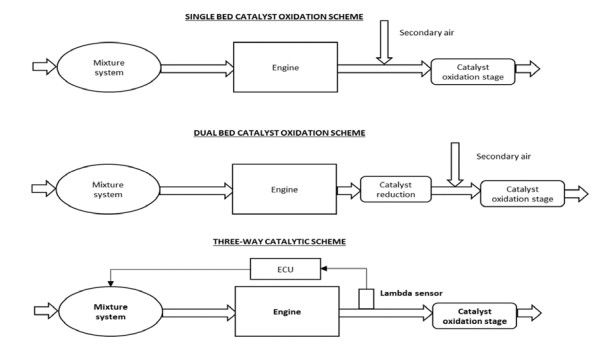
|
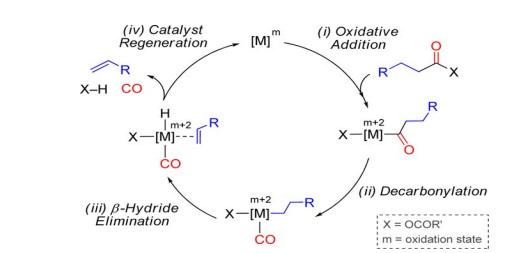
|
The Role of Metal in Health and Medicine
Metal complexes have been used for health and healing for eras, primarily due to their antibacterial and antiviral therapeutic potential. Gold-based drugs were utilized in China and the Middle East as far back as 3,500 years ago. Metals such as Platinum, lithium, Zinc, Silver, Cu and Bismith treat various conditions, from heartburn and arthritis to cancers, tumours, viral infections and depression[33]. Gold compounds are still the only pharmaceuticals that can pause the progression of rheumatoid arthritis[39]. Disinfection of drinking water was achieved by storing it in silver pots during the Persian Empire, and Hg2Cl2 was an active diuretic during the Renaissance period[40]. Gadolinium is used as a contrast agent in MRI scans[41]. Gold and silver have antimicrobial properties and are used in medical implants and wound dressings[42]. Certain heavy metals, like platinum, are used in chemotherapy drugs to treat cancers. Platinum-based drugs, such as cisplatin, effectively target and destroy cancer cells[43]. Several treatments for most widespread diseases have been based on inorganic drugs. The metal can increase the chemotherapeutic properties of organic molecules such as thiosemicarbazones[44]. Metals can form complex compounds with different coordination numbers, geometries, diverse oxidation numbers, and the ability to bind to biomolecules. These features prove the potential of metal ions as an exciting means to explore innovation in drug discovery[37].
Today, metals known as theranostics characterize the roles of therapeutics and diagnostics. Magnetic nanoparticles (MNPs), typically composed of metal oxides, can adjust the magnetic field in their surroundings. This ability enhances their use in theranostic nanomedicine, owing to their potential for heat generation and actuation.[45]. In recent years, the application of magnetic nanomedicine as a theranostic device has attracted enormous attention in cancer treatment research where magnetite Fe3O4 functionalized with the Re(I) tricarbonyl complexes as a bimodal contrast agent for MRI and optical imaging of nanoparticles (Fig.9)[45-50]. A bimodal contrast agent is an imaging agent used in therapeutic diagnostics that offers two dissimilar forms of contrast or imaging signals. These agents are designed to boost the perceptibility of structures or processes within the body using two distinct imaging modalities, like magnetic resonance imaging (MRI) and optical imaging, or MRI and positron emission tomography (PET). The dual functionality allows for more comprehensive and accurate diagnostic information, combining the strengths of both imaging techniques. The reclaimed and growing interest in metal complexes' antimicrobial effect is related to antibiotic resistance, which signifies a health and social problem worldwide and is a significant hazard for humankind soon, as recently recognized by the World Health Organization.
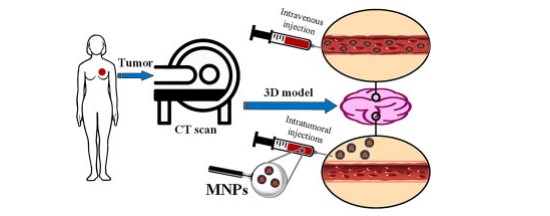
|
Environmental and Health Implications
Heavy metal poisoning is associated with the accumulation of heavy metals in significant amounts in the body's soft tissues[43]. Many heavy metals, including Zn, Cu, Cr, and Fe, are required by living organisms, including plants, in trivial quantities, while excesses cause severe damage to the body. The heavy metals Pb, Hg, As and Cd have no biological functions, but their presence in the environment is due to the waste discharge as a result of industrial acquaintance, air or water contamination, foods, medicines, inadequately coated food containers, or the assimilation of lead-based paints are the source for accretion of it in the body results in severe health issues[46-55].
Environmental Monitoring and Remediation
Air, soil, water, and noise are the critical factors for a monitoring environment related to the impact of heavy metal discharge. Technologies have been developed to remediate environments contaminated with heavy metals. Phytoremediation, bioremediation using microorganisms, for example, uses plants to absorb and extract heavy metals from the soil and microbes to accumulate metals[49]. Additionally, advanced filtration systems can remove heavy metals from water sources.
Pollution and Toxicity
Heavy metal poisoning is related to the exposure, period and type of metal. It also depends upon its oxidation states as the Cr trivalent is non-toxic. At the same time, Cr (VI) is toxic for plant development, while alteration in Ca accumulation in the presence of Pb is also related to oxidation states[56]. Heavy metals pose serious health risks when they spread in the environment faced by the human body[49] and affect humans in the same way as they do regarding exposure time. Lead, mercury, cadmium, and arsenic are notorious for lethal effects[51]. These metals can contaminate soil, water, and air, leading to adverse health effects like neurological damage, kidney failure, respiratory issues, and even cancer[50]. Pb toxicity distresses adults less often than children[50]. In the last two decades, statistics have shown that Pb is harmful to children, especially in the blood. Specific examples of heavy metals (nonbiological role) contribution to world advancement and toxicity are discussed below:
Bioaccumulation of heavy metals
Heavy metals have ability to bioaccumulate in the food chain, meaning they can build up in organisms over time. For instance, mercury released into the environment can transform into methylmercury, accumulating in fish and shellfish. When humans consume these contaminated seafood products, they risk mercury poisoning.
Pb significance and poisonous
Lead (Pb) production workers, battery plant workers, welders and soldiers may be overexposed to Pb if appropriate precautions are not taken[56-65]. Pb is stored in the bone but may affect any organ system. The effects of Pb poisoning vary depending on the age of the specific and the quantity of experience. In children, symptoms vary depending on the degree of exposure to Pb. Approximately affected individuals may not have any noticeable signs. Symptoms usually develop over a three to six-week time period. In children, overexposure to Pb may cause less power for lively, incompetent, short-tempered, and inactive (tired). Some other symptoms also include lack of appetite (anorexia), constipation, headaches, vomiting, abdominal pain, slurred speech (dysarthria), variations in kidney function, considerable quantity of protein in the blood (hyperproteinemia), and ashen skin (pallor) ensuing a low amount of iron in the red blood cells causes anemia[58]. Neural signs of Pb overexposure include a reduced capability to manage voluntary movements, brain damage (brain disorder), annexations, shaking, papilledema, and/or impaired perception. Some affected children experience learning or behavioural problems such as mental retardation and selective discrepancies in verbal communication, cognitive function, balance, behaviour, and school presentation. In some cases, symptoms may be life-threatening[59-65]. Pb may affect adults and cause blood pressure, reduced muscles strength and endurance, kidney disease, wrist drop, and behavioural changes such as hostility, depression, anxiety and damage to the reproductive organs, which may be life-threatening. Additional symptoms may include listlessness, difficulty in sleeping (insomnia), fever, headaches, fatigue, exhaustion, vomiting, loss of appetite (anorexia), abdominal pain, impairment, joint pain, loss of newly acquired skills, incoordination, irritability, altered consciousness, hallucinations, or seizures. In addition, affected individuals may experience low iron levels in the red blood cells (anemia), marginal neuropathy, and, roughly in a few cases, brain damage. Lead (Pb) is primarily expelled through urine and feces, but it can also be found in hair, nails, sweat, saliva, and breast milk and could be dangerous.
Hg significance and poisonous
Hg is only a liquid metal used extensively in thermometers, barometres and manometres due to its high density. Hg is also used as a liquid mercury electrode in manufacturing Cl and NaOH by electrolysis of Brine. Mercury compounds have many uses. Calomel (mercurous chloride, Hg2Cl2) is a standard in electrochemical measurements and medicine as a cleansing. Mercuric Chloride (HgCl2), a corrosive sublimate, is used as an insect repellent, in rat poison, and as an antiseptic. Skin ointments contain mercuric oxide, and mercuric sulphate is frequently used as a catalyst in organic chemistry. Vermilion, a red pigment, is composed of mercuric sulphide, while another crystalline form used as a pigment appears black. Mercury fulminate (Hg(CNO)₂) serves as a detonator. Mercury is still utilized in certain electrical devices, including switches and rectifiers, which must be reliable for industrial catalysis. The amount of Hg used in consumer batteries and fluorescent lighting has significantly decreased. Mercury is used by dental assistants, hygienists, and chemical workers. The toxicity of Hg can affect the lungs, kidneys, brain, and skin. Symptoms of mercury poisoning include disruption of the nervous system, damage to the brain, fatigue, depression, lethargy, irritability, and headaches[56-63]. Respiratory symptoms associated with inhalation of Hg vapours include respiratory distress, coughing, dyspnea, tightness or burning pain in the chest. Some affected individuals may experience an abnormal fluid buildup in the lungs called pulmonary edema, pneumonia, and/or irregular creation of fibrosis[61-69]. Behavioural and neurological changes may be associated with overexposure to mercury poisoning, such as lack of concentration, excitability, loss of memory and quick-tempered behaviour. Mercury poisoning can also lead to shock and irreversible brain damage. Few pretentious people observe psychological misperception. A progressive cerebellar syndrome may also occur due to overexposure to Hg, causing ataxia, impaired coordination of voluntary arm movements. Abnormal involuntary body movements, such as uncontrolled irregular and slow wriggling, are also common symptoms of Hg. Other symptoms comprise non-inflammatory deteriorating sickness of the nerves (polyneuropathy); reduced ability to manage charitable activities (cerebellar ataxia); legs and arms anxieties while, in some cases, of the tongue and lips; seizures; and slurred speech (dysarthria). Mood, behaviour, and consciousness changes may also occur[62,69].
In a few cases, lingering contact with inorganic Hg, a personality disorder known as erethism or Madhatter syndrome, may observed. Symptoms allied with Mad Hatter syndrome include retention damage, extreme nervousness, irregular edginess, or insomnia. This syndrome was defined in workers exposed to mercury in the felt-hat industry. Many affected individuals experience corporeal losses such as visual problems (e.g. tightening of visual fields, tunnel vision, and blindness) and hearing injury. Some persons may experience skin changes, such as painful swelling and pink discoloration of the fingers and toes (acrodynia), insistent soreness or inflammation of the skin (erythema), extreme sensitivity (hyperesthesia) in the affected areas, and tingling or sensory disturbances.
In some cases, affected individuals may also experience gastrointestinal disorders, damage to the kidney, dryness, failure of acute renal, inflammation of the gums (gingivitis), severe local frustration of the mouth and pharynx, vomiting, and abdominal cramps accompanied by bloody diarrhea. Mercury is primarily excreted through urine and feces.
Cd significance and poisonous
The use of Cadmium (Cd) metal is evident in textiles, batteries, ceramics, electronics, metal-finishing industries, electroplating industries, pigments, petroleum products, insecticides, solders, television sets, metallurgical industries, steel, photography, plastics, and synthetic chemicals having no biological functions in living organism[3,50-55]. The discharge of waste from these industries also contains Cd metal; therefore, it is apparent that its presence in soil or running streams raises health issues. Using soil or contaminated water in agriculture will lead to the accumulation of non-essential metals in plants, which humans use as food, leading to progressive loss of lung function and other diseases[53]. Overexposure may lead to symptoms such as fever, fatigue, nausea, vomiting, abdominal cramps, diarrhea, and headaches. Consequently, it can result in progressive lung damage, including emphysema, fluid buildup in the lungs (pulmonary edema), and shortness of breath (dyspnea).
In some cases, affected individuals may experience increased salivation, yellowing of the teeth, rapid heartbeat (tachycardia), anemia (low iron levels in red blood cells), skin become bluish and mucous membranes (cyanosis) due to lack of oxygen, and a reduced sense of smell (anosmia). Cadmium poisoning can also cause kidney dysfunction, characterized by rich protein levels in the urine (proteinuria), mild liver function changes, and bone softening (osteomalacia).[44,49,56]. Cd is also toxic to the aquaculture industry, where the growth of the fish is constrained and unhealthy for food.
As significance and poisonous
Arsenic (As) is an essential pesticide, herbicide, insecticide, cotton desiccant, defoliant, and soil sterilant in the agricultural industry. They are also used in catalysts, pyrotechnics, antifouling agents in paints, pharmaceutical substances, dyes and soaps, ceramics, alloys (automotive solder and radiators), and electrophotography. As and its compounds are used in pharmaceuticals like leukemia, psoriasis, chronic bronchial asthma, wood preservatives (chromated copper arsenate), agricultural chemicals, mining, metallurgical, glass-making, and semiconductor applications[69]. Chromated copper arsenate is no longer used in residential applications following a voluntary ban on its use in Canada and the United States of America at the end of 2003. During 1990–2002, approximately 4% of arsenic produced was used to manufacture glass, and 1–4% was used in producing non-ferrous alloys[70]. Organic forms of arsenic were constituents of some agricultural pesticides in the USA. However, in 2009, the U.S. Environmental Protection Agency issued a cancellation order to eliminate and phase out the use of organic arsenic pesticides by 2013[70]. Other organic arsenicals (e.g. roxarsone, arsanilic acid and its derivatives) are used as feed additives for poultry and swine to increase weight gain and improve feed efficiencies, pigmentation, and disease treatment and prevention[71]. Arsenic and arsenic compounds are used for a variety of other industrial purposes. Elemental arsenic is used to manufacture alloys, particularly with lead (e.g. in lead acid batteries) and copper. Gallium arsenide and arsine are widely used in the semiconductor and electronics industries. Because of its high electron mobility, as well as light-emitting, electromagnetic and photovoltaic properties, gallium arsenide is used in high-speed semiconductor devices, high-power microwave and millimetre-wave devices, and opto-electronic devices, including fibre-optic sources and detectors[74]. Arsine is a doping agent to manufacture computer chips and fibre optics crystals. Arsenic and arsenic compounds manufacture pigments, sheep dips, leather preservatives, and poisonous enticements[70-75].
CONCLUSION
The significance of heavy metals in daily life is multifaceted. While they are indispensable in various industries and technologies, their potential toxicity requires careful management and regulation to mitigate environmental and health risks. Understanding and balancing their beneficial uses against their hazardous impacts is crucial for supportable development and public health. A sustainable and effective program is needed to prevent heavy metal toxicity while pursuing its importance for global progress.
ACKNOWLEDGEMENT
Author is thankful to the net services provided by the University of Karachi and Jinnah University for Women Karachi, Pakistan
REFERENCES
- https://www.lenntech.com/processes/heavy/heavy-metals/heavy-metals.htm
- P.B. Tchounwou, Yedjou, C. G., Patlolla, A. K., & Sutton, D. J.,Environmental toxicology.Molecular, clinical and environmental toxicology, Experientia Supplementum,3; 133-164. (2012).
- W. T. Kwong, Friello, P., & Semba, R. D..Sci. Total Environ.,330(1-3); 21-37,(2004).
- G. Kazantzis, Biometals,17; 493-498, (2004).
- A. Bhan, Sarkar N.N. Rev. Environ. Health,. 20; 39-56, (2005).
- C. S. Cao, Shi, Y., Xu, H., & Zhao, B.Coordinat. Chem. Reviews,365; 122-144, (2018).
- R. H. Crabtree, The organometallic chemistry of the transition metals. John Wiley & Sons. (2009).
- M. Jaishankar, Tseten,T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. Interdiscip. Toxicol. 7: 60–72. (2014).
- P. Dudeja, K., Grover, S., Sıngh, H., & Jabin, Z. Europ. Oral Research,57(2), 60-67. (2023).
- R. A. Powers, Batteries for low power electronics.Proceedings of the IEEE,83(4), 687-693. (1995).
- I. Råde, & Andersson, B. A.J. power Sources,93(1-2); 55-71,(2001).
- J. McDowall, Proceedings of the Conference (Cat. No. 99TH8371)(pp. 303-308). IEEE. (1999)
- L. Ciacci, L., Fishman, T., Elshkaki, A., Graedel, T. E., Vassura, I., & Passarini, F.Global Environ. Change,63, 102093 (2020).
- https://www.statista.com/statistics/1339880/global-battery-market-size-by-technology/
- https://www.americanchemistry.com/chemistry-in-america/news-trends/blog-post/2018/transition-metals-and-their-many-uses
- https://www.civmats.com/news/news46/news46.HTML
- https://www.electrispower.com/blog/copper-in-the-construction-and-architecture-industry
- https://www.rsc.org/periodic-table/element/26/iron#:~:text=90%25%20of%20all%20metal%20that,girders%20etc)%20and%20in%20manufacturing.
- https://www.alamy.com/cartoon-construction-building-materials-piles-of-sand-cement-and-stones-red-brick-stacks-wooden-planks-and-metal-roof-elements-for-house-building-industry-and-renovation-vector-set-image459838661.html
- https://www.designingbuildings.co.uk/wiki/Metal_in_construction#:~:text=Aluminium%20wall%20cladding%20systems%20are,
%2C%20spandrels%2C%20and%20so%20on. - R. Price, Dyes and Pigments. In: Wilkinson, G. (ed.) Comprehensive Coordination Chemistry, 35–94. Pergamon, Oxford (1987).
- J. N. Chakraborty, J. N. Metal-complex dyes. InHandbook of textile and industrial dyeing. 446-465. Woodhead Publishing.(2011).
- https://www.worlddyevariety.com/reactive-dyes/reactive-violet-1.html
- M. H. Vijaykumar, Veeranagouda, Y., Neelakanteshwar, K., & Karegoudar, T. B.World J. Microb. & biotech,22,;157-162, (2006).
- https://vibron.com.au/organic-pigments-for-plastics/
- https://link.springer.com/referenceworkentry/10.1007/978-1-4419-8071-7_197/figures/425
- https://bioorganics.biz/product_details.php?id=203566
- R. L. M. Allen, Colour Chemistry. London: Thomas Nelson and Sons Ltd. pp. 11–.13, (1971).
- J. R. Aspland, Textile Dyeing and Coloration. Association of Textile Chemists and Colorists. 3–310 (1997).
- S. Velusamy, Roy, A., Sundaram, S., & Mallick, T. K. The Chemical Record,21(7);1570-1610, (2021).
- E. Kritsanaviparkporn., Baena-Moreno, F. M., & Reina, T. R.Chemistry,3(2); 630-646, (2021).
- M. A. Ortuño, Dereli, B., & Cramer, C. J.Inorg. Chemist.,55(9); 4124-4131, (2016).
- I. G. Medina-Meza,., Barnaba, C., & Barbosa-Cánovas, G. V. A review.Innovative Food Sci & Emerg. Tech.22; 1-10, (2014).
- E. Sher, Handbook of air pollution from internal combustion engines: pollutant formation and control. Academic press. (1998).
- J. A. Melo-Banda, J. A., Lam-Maldonado, M., Rodríguez-Gómez, F., Hérnandez-Vega, L. K., Malpica-Maldonado, J. J., & de la Torre, A. R.,Catalysis Today,392;72-80, (2022).
- D..M. Aruguete, D. M., Murayama, M., Blakney, T., & Winkler, C..Environ. Sci. Processes & Impacts,21(1); 133-144. (2019).
- J. Rinkovec, Arhiv za higijenu rada i toksikologiju,70(4); 224-230. (2019).
- D. Tesauro, Int.J. Mol. Sci.23(8), 4377. (2022).
- P. Dolara, Int.J..Food Sci. & Nutri.,65(8); 911-924, (2014).
- E. R. Tiekink,Gold Bulletin,36(4); 117-124, (2003).
- M. D. Sobsey, Stauber C. E, Casanova, L. M, Brown J. M, Elliott M.A. Environ Sci Technol.42(12); 4261–7. (2008).
- https://www.insideradiology.com.au/gadolinium-contrast-medium/
- M. Konop, M., Damps, T., Misicka, A., & Rudnicka, L. a minireview, J. of Nanomaterials,2016(1); 7614753, (2016).
- C. Zhang, Xu, C., Gao, X., & Yao, Q.Theranostics,12(5); 2115, (2022).
- E.. J. Siddiqui, Azad, I., Khan, A. R., & Khan, T. a review.J. of drug delivery & therapeutics,9(3); 689-703, (2019).
- O. L Gobbo., Sjaastad, K., Radomski, M. W., Volkov, Y., & Prina-Mello, A.Theranostics,5(11); 1249, (2015).
- M. Mkhatshwa, Moremi, J. M., Makgopa, K., & Manicum, A. L. E..Inter. J of Mole. Sci.,22(12); 6546. (2021).
- S. Carron, Bloemen, M., Vander Elst, L., Laurent, S., Verbiest, T., & Parac-Vogt, T. N..J.of Mat. Chem. B,3(21); 4370-4376, (2015).
- A. Dahaghin, Emadiyanrazavi, S., Salimibani, M., Bahreinizad, H., Haghpanahi, M., Eivazzadeh-Keihan, R., & Maleki, A. Biocybernetics and Biomed. Eng.41(2), 516-526, (2021).
- Z. L. He, Yang X. E. , Stoffella P. J. J Trace Elem. Med Biol.;19(2–3):125–140 (2005)
- C.O Abernathy, Liu, Y. P., Longfellow, D., Aposhian, H. V., Beck, B., Fowler, B., ... & Waalkes, M..Environ.. health perspect,107(7), 593-597 (1999).
- S. Hayat, Khalique G., Irfan M., Wani A.S., Tripathi B.N., Ahmad A. An Overview.Protoplasma., 249; 599–611, ( 2012).
- L. Huang, Yu, C. H., Hopke. P. K., Shin J.Y., Fan Z.J. Air Waste Manag. Assoc.64;1439–1445, (2014)
- S.Ur Rahman, Qin, A., Zain, M., Mushtaq, Z., Mehmood, F., Riaz, L., Naveed, S., Ansari, M. J., Saeed, M., Ahmad, I., & Shehzad, M. A review. Heliyon, 10(6;, e27724, (2024).
- Gesamp.IMO/FAO/UNESCO/WMO/WHO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Pollution: Report of the seventeenth session.Geneva, Switzerland: World Health Organization;. (Reports and Studies No. 31), (1987)
- D.N.Wilson, Association Cadmium. Cadmium market trends and influences; London. Cadmium 87 Proceedings of the 6th Inter. Cd. Conf.. pp. 9–16, (1988).
- U.S Environmental Protection Agency (EPA)[accessed 4 March 2009]; Cadmium Compounds.(2006)
- D.C. Paschal, Burt V, Caudill S.P, Gunter E.W, Pirkle J.L, Sampson E.J, et al., 1988–1994.Arch. Environ. Contam. Toxicol.38; 377–383, (2000).
- P.N. Gabby.Lead: in Mineral Commodity Summaries.Reston, VA: U.S. Geological Survey; 2006.
- B. P. Lanphear, Matte T.D., Rogers J, Clickner, R. P., Dietz, B., Bornschein, R. L., ... & Jacobs, D. El..Environ Res., 79; 51–68, (1998).
- D. E. Jacobs, Clickner R.P., Zhou J.Y., Viet, S. M., Marker, D. A., Rogers, J. W., ... & Friedman, W., Environ. Health Perspect.110; A599–A606,(2002).
- Agency for Toxic Substances and Disease Registry (ATSDR)Case Studies in Environmental Medicine - Lead Toxicity.Atlanta: Public Health Service, U.S. Department of Health and Human Services; (1992)
- K. L., Nuttall. Ann. Clin. Lab. Sci.;34;235-50. (2004)
- T. W., Clarkson, Magos, L., & Myers, G. J.,New England J. of Med..,349(18); 1731-1737, (2003)
- F. Zahir, Rizwi, S. J., Haq, S. K., & Khan, R. H.Environ. Toxicol.& pharmacol.,20(2); 351-360, (2005).
- M. Valko, Rhodes, C. J. B., Moncol, J., Izakovic, M. M., & Mazur, M..Chemico-biolo, interactions, 160(1); 1-40, (2006).
- A.S. Andrew, Burgess J. L, Meza M.M., Demidenko, E., Waugh, M. G., Hamilton, J. W., & Karagas, M. R.Environ Health Perspect, 114;1193–1198. (2006)
- https://www.federalregister.gov/
- https://www.epa.gov/archive/epa/aboutepa/epa-2009-progress-report.html
- https://archive.epa.gov/emap/archive-emap/web/html/index-124.html
- ATSDR. Toxicological Profile for Arsenic. Atlanta, Georgia. (2007).
- L. Benbrahim-Tallaa, Webber, M. M, Waalkes M. P.Environ Health Perspect.,115;243–247, (2007)
- J. Liaw, Marshall G, Yuan Y, Ferreccio, C., Steinmaus, C., & Smith, A. H.,Cancer Epidemiol Biomarkers Prev. 17:1982–1987, (2008)
- M. Kumar, M., Seth, A., Singh, A. K., Rajput, M. S., & Sikandar, M., Remediation strategies for heavy metals contaminated ecosystem: A review.Environ. & Sustain. Indicators,12; 100155, (2021)
- https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Inorganic-And-Organic-Lead-Compounds-(2006)
How to Cite this paper?
APA-7 Style
Nasreen,
H., Haider,
S. (2024). A Review of the Significant Role of Heavy Metals in the Advancement of the World and its Contribution to Pollution. Pakistan Journal of Chemistry, 14(3-4), 49-59. https://doi.org/10.15228/2024.v14.i03-4.p06
ACS Style
Nasreen,
H.; Haider,
S. A Review of the Significant Role of Heavy Metals in the Advancement of the World and its Contribution to Pollution. Pak. J. Chem. 2024, 14, 49-59. https://doi.org/10.15228/2024.v14.i03-4.p06
AMA Style
Nasreen
H, Haider
S. A Review of the Significant Role of Heavy Metals in the Advancement of the World and its Contribution to Pollution. Pakistan Journal of Chemistry. 2024; 14(3-4): 49-59. https://doi.org/10.15228/2024.v14.i03-4.p06
Chicago/Turabian Style
Nasreen, H., and S. Haider.
2024. "A Review of the Significant Role of Heavy Metals in the Advancement of the World and its Contribution to Pollution" Pakistan Journal of Chemistry 14, no. 3-4: 49-59. https://doi.org/10.15228/2024.v14.i03-4.p06

This work is licensed under a Creative Commons Attribution 4.0 International License.


